LAHORE – A major public health alarm has been raised after Directorate of Drugs Control Authority Punjab ordered the immediate ban and recall of eight different medicines found to be dangerous, substandard, and potentially life-threatening.
According to official alert, the affected medicines failed critical quality and potency tests, while some were found to be adulterated and illegally labeled, violating drug safety laws. Authorities have warned that continued use of these medicines could pose serious risks to human health.
All pharmacies, wholesalers, and distributors have been instructed to stop selling these medicines at once and submit full stock details to concerned drug inspectors. Dealers in possession of the affected batches have been ordered to return them immediately.
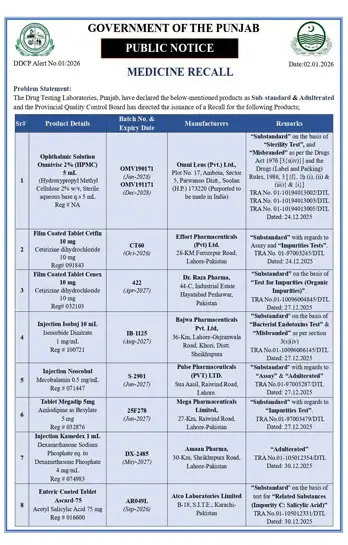
| Sr# | Product Details | Batch No. & Expiry Date | Manufacturers | Remarks |
| 1 | Ophthalmic Solution Omnivise 2% (HPMC) 5 mL (Hydroxypropyl Methyl Cellulose 2% w/v, Sterile aqueous base q s 5 mL) Reg# NA | OMV1908173 (Jun-2023) OMV1912171 (Dec-2023) | Omni Lens (Pvt.) Ltd., Plot No. 17, Audhata, Sector 5, Pantnagar, Distt. Udham Singh (U.P.) 173220 (Purported to be made in India) | “Substandard” on the basis of “Sterility Test”, and “Misbranded” as per the Drugs Act 1976 $S$ 3(j)(vi) and the Drugs (Labeling and Packing) Rules, 1986, $S$ 3(f), 10, 11(ii) & 11(iii)(a). TRA No. 01-101194013002/DTL TRA No. 01-101194013003/DTL TRA No. 01-101194013005/DTL Dated: 24.12.2025 |
| 2 | Film Coated Tablet Cetifin 10 mg Cetirizine Dihydrochloride 10 mg Reg# 091843 | CT60 (Oct-2026) | Effort Pharmaceuticals (Pvt) Ltd. 28-KM Ferozepur Road, Lahore-Pakistan | “Substandard” with regards to Assay and “Impurities Tests”. TRA No. 01-97003245/DTL Dated: 24.12.2025 |
| 3 | Film Coated Tablet Cenez 10 mg Cetirizine dihydrochloride 10 mg Reg# 032103 | 422 (Apr-2027) | Dr. Raza Pharma, 44-C, Industrial Estate Hayatabad, Peshawar, Pakistan | “Substandard” on the basis of “Test for Impurities (Organic Impurities)”. TRA No. 01-10096004845/DTL Dated: 24.12.2025 |
| 4 | Injection Isobaj 10 mL Isosorbide Dinitrate 1 mg/mL Reg# 030721 | IB-1125 (Aug-2027) | Bajwa Pharmaceuticals Pvt. Ltd. 36-Km, Lahore-Okara/wala Road, Kheri, Distt. Sheikhupura | “Substandard” on the basis of “Bacterial Endotoxins Test” & “Misbranded” as per section 3(j)(v). TRA No. 01-10096006145/DTL Dated: 27.12.2025 |
| 5 | Injection Neurobal Mecobalamin 0.5 mg/mL Reg# 071447 | S-2001 (Jun-2027) | Pulse Pharmaceuticals (Pvt.) LTD. Sua Asal, Kasur Road, Kot Lahore. | “Substandard” with regards to “Assay” & “Adulterated”. TRA No. 01-97017287/DTL Dated: 27.12.2025 |
| 6 | Tablet Megadip 5mg Amlodipine as Besylate 5 mg Reg# 032876 | 25F278 (Jun-2027) | Mega Pharmaceuticals Limited, 27-Km, Raiwind Road, Lahore-Pakistan | “Substandard” with regards to “Impurities Test”. TRA No. 01-97001408/DTL Dated: 27.12.2025 |
| 7 | Injection Kamedex 1 mL Dexamethasone Sodium Phosphate eq. to Dexamethasone Phosphate 4 mg Reg# 074983 | DX-2485 (May-2027) | Aman Pharma, 30-Km, Sheikhupura Road, Lahore-Pakistan | “Adulterated”. TRA No. 01-105012354/DTL Dated: 30.12.2025 |
| 8 | Enteric Coated Tablet Ascard 75 Acetyl Salicylic Acid 75 mg Reg# 016600 | AR049L (Sep-2026) | Alco Laboratories Limited B-18, S.I.T.E. Karachi-Pakistan | “Substandard” on the basis of test for “Related Substances (Impurity C: Salicylic Acid)”. TRA No. 01-105012331/DTL Dated: 30.12.2025 |
Health officials have strongly advised the public to discontinue use of these medicines without delay, calling them extremely hazardous.
Officials warned avoiding handshakes if suffering from cold or flu. Maintain physical distance. Wear masks. Strengthen immunity through a healthy diet.
Authorities say strict legal action will be taken against those found violating the recall order, as the government intensifies efforts to protect public health.
Pre-Departure Desks rolled out across Pakistani airports amid Crackdown