ISLAMABAD – A public safety health update shared by Drug Regulatory Authority of Pakistan (DRAP) which recalled famous antibiotic syrups along with contraceptive medicine produced by Zafa Pharmaceuticals.
Patients currently using any GlaxoSmithKline (GSK) Amoxicillin Forte are advised to consult their healthcare providers for alternatives.
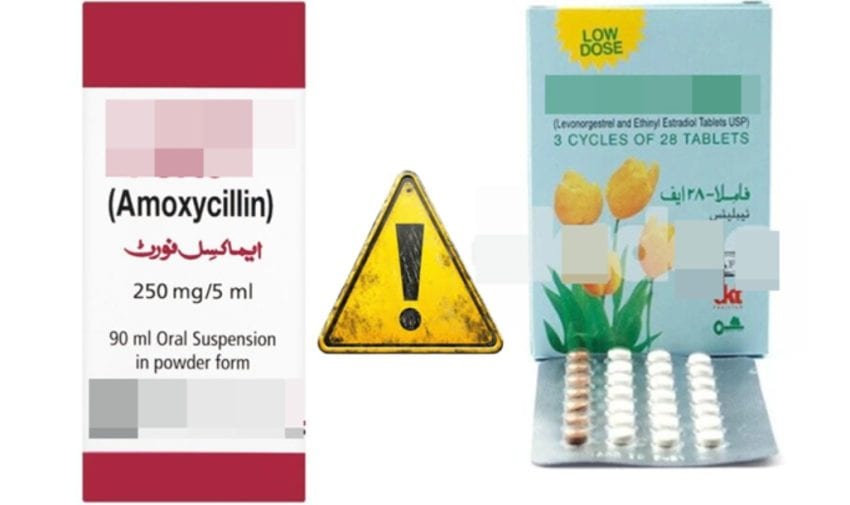
DRAP said Amoxil Forte 250mg and Amoxil Forte 125mg syrups are commonly prescribed for infections such as typhoid and meningitis are being withdrawn from hospitals, pharmacies, and medical distributors nationwide.
The action follows discovery of packaging defects, particularly issues with bottle caps and seals, which could compromise the safety and effectiveness of the medicines.
A total of 58 batches of Amoxil Forte 250mg and 111 batches of Amoxil Forte 125mg have been marked for immediate recall. The problem was first reported by GSK itself, prompting DRAP to act swiftly.
DRAP mentioned that corrective action was taken as soon as manufacturer alerted us, and that DRAP
is committed to protect public health and ensuring medicine quality. Healthcare facilities have been instructed to identify and remove the affected batches without delay.
DRAP also ordered immediate market withdrawal of contraceptive tablet Famla 28F manufactured by Zafa Pharmaceuticals. Central Drugs Laboratory in Karachi found the batches to be non-compliant with quality standards during routine surveillance testing.