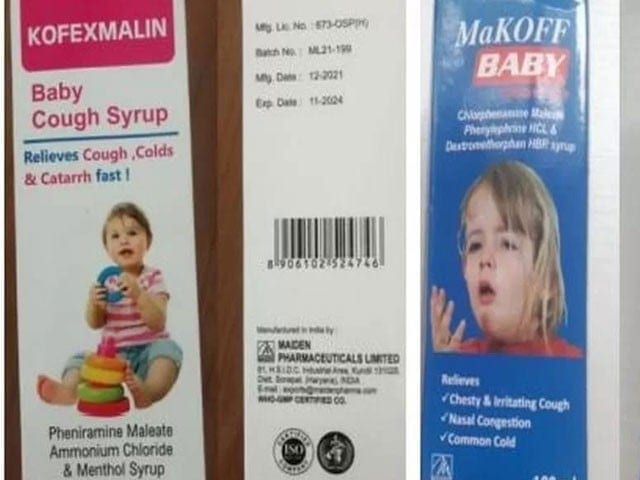
BANJUL – The Gambian government is exploring avenues for legal action against an Indian drug maker and a local distributor over toxic cough syrups, which caused deaths of dozens of children in the African country.
At least 70 Gambian children, most of them under 5, died from acute kidney injury last year. A Gambian government task force investigated the deaths and found that they were “a direct result” of the contaminated cough and cold syrups imported from India.
Previously, Gambia fired the executive director and deputy director of its Medicines Control Agency (MCA) and referred the matter to police after the task force found that the medicines in question were not registered with the MCA, which is required by law.
Now the small West African country has hired a US law firm to explore legal action against India and its drug maker Maiden Pharmaceuticals.
Maiden Pharmaceuticals has denied wrongdoing, and the Indian government says that tests it conducted on the drugs showed they were not contaminated.
Families of 20 of the children have already sued the two companies as well as Gambian authorities.
“The government is currently benefiting from legal advice from a top tier international law firm,” Gambia’s government said in the statement, adding it is pursuing potential redress through engagement with the Government of India.
It also said that the health ministry has hired a firm that is reviewing all the health-related legislation in the Gambia.