ISLAMABAD – The Drug Regulatory Authority of Pakistan (DRAP) has imposed a ban on the sale and use of the blood disorder injection, Rhophylac.
It has issued a product recall alert, advising against the use of the human anti-D immunoglobulin drug as the sample label of the injection contains incorrect details.
The counterfeit drug was reported by the provincial drug inspector and a pharmaceutical company in Karachi.
Rhophylac injection is available in medicine markets across the country. However, the sample packets of the injection carry two different batch numbers as it surfaced after the barcodes printed on them were scanned.
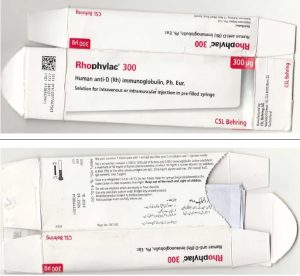
According to DRAP, anti-D immunoglobulin is a commercial biological antibody derived from human blood plasma. It targets the patient’s red blood cells, and is used for treating a condition known as “Immune Thrombocytopenic Purpura” (ITP). The use of counterfeit injections can be extremely harmful to health.
DRAP’s alert emphasized that counterfeit medicines have unclear quality and efficacy, and therefore should not be used.