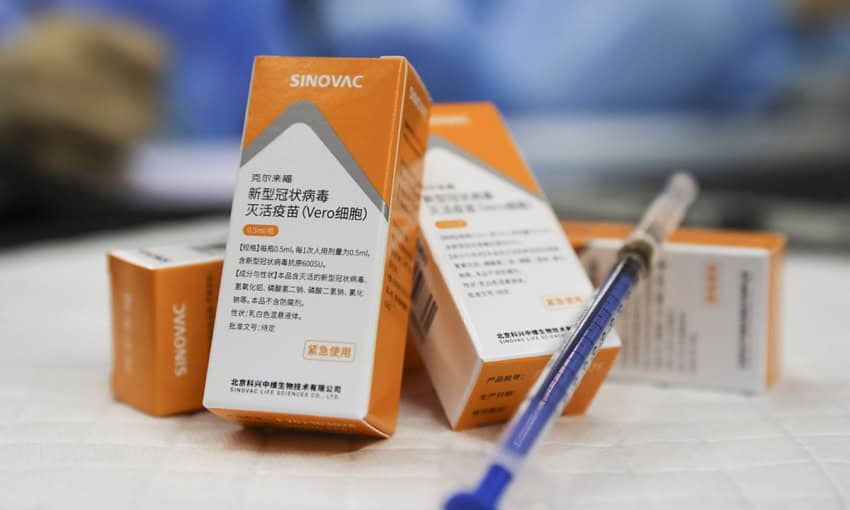
The World Health Organization (WHO) on Tuesday approved the China-made vaccine, Sinovac, for emergency use against COVID-19.
This is the second Chinese drug approved by the global health body for use against the virus that has claimed more than 3.55 million lives across the world.
“WHO today validated the Sinovac-CoronaVac Covid-19 vaccine for emergency use, giving countries, funders, procuring agencies and communities the assurance that it meets international standards for safety, efficacy and manufacturing,” the UN health agency said in a statement.
Pakistan is also administrating jabs of Sinovac vaccine besides two other Chinese vaccines – Sinopharm and single-dose Convidecia vaccine developed by Cansino Biologicals.
Earlier today, Pakistan launched locally-processed single dose COVID-19 vaccine ‘PakVac’ amid the government’s efforts to pace up the vaccination drive of the country.
Speaking on the occasion, Special Assistant to Prime Minister (SAPM) on Health Dr Faisal Sultan said the country will soon be able to start the production of vaccine.
Praising Chinese assistance for Pakistan in the health crisis, he said that China was a strong friend of the country. He also appreciated the National Institute of Health (NIH) teams for successfully developing the vaccine from the raw materials given by China.
Meanwhile, National Command and Operation Centre (NCOC) chief Asad Umar termed the day “important”. He said that the number of patients hospitalised during the third wave of the pandemic was higher than the number of patients who were hospitalised during the first wave.
He further said that efforts were being made to strengthen the national health system.
Chinese Ambassador Nong Rong, speaking on the occasion, said that production of the vaccine depicted level of friendship between the two countries.
Pakistan approves Pfizer coronavirus vaccine for emergency use